| Name | Vonoprazan |
|---|---|
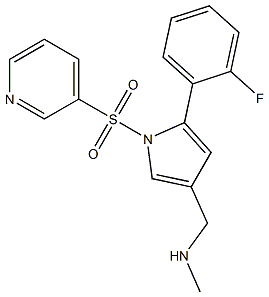
| Synonyms | Vonoprazan; 1-[5-(2-Fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrol-3-yl]-N-methylmethanamine; 1H-Pyrrole-3-methanamine,5-(2-fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl); TAK-438 (free base); Vonoprazan-025; Voronazan interfluoroisomer; Vonoprazan related Impuirty 29 |
| CAS NO | 881681-00-1 |
| Molecular Weight | 345.3912432 |
| Molecular Formula | C17H16FN3O2S |
| Mol File | 881681-00-1.mol |
Vonoprazan Chemical Properties | |
|---|---|
| Boiling point | 530.3±60.0 °C(Predicted) |
| density | 1.31±0.1 g/cm3(Predicted) |
| CAS DataBase Reference | 881681-00-1 |
| pka | 9.06±0.10(Predicted) |
| Vonoprazan Usage And Synthesis | |
|---|---|
| Indications and Uses | Vanoprazan fumarate is a new oral gastric acidity drug developed by Takeda Pharmaceutical and Otsuka Pharmaceuticals. It is used to treat duodenal ulcers, gastric ulcers, reflux esophagitis, gastric ulcers or recurrent duodenal ulcers caused by low dosages of Aspirin, and Helicobacter pylori. It can also supplement treatment of gastric ulcers, duodenal ulcers, gastric MALT lymphoma, idiopathic thrombocytopenic purpura, early gastric cancer, and Helicobacter pylori infection gastritis. Compared to traditional irreversible proton pump inhibitors (omeprazole, esomeprazole, etc.), Takecab has the following advantages: 1. Rapid effects, having the most drastic acid-suppressing effects on the first day of ingestion. 2. Oral intake, efficacy unaffected by gastric acid, does not require enteric administration. Alleviated nighttime acid reflux. |
| Mechanisms of Action | Takecab (TAK-438) is a kind of potassium ion (K+) competitive acid blocker (P-CAB) and a reversible proton pump inhibitor. After this product enters the body, at the last step of gastric parietal cell acid secretion, it inhibits K+ ions from bonding with H+-K+-ATP enzymes (proton pump), thus stopping gastric acid secretion. It has a strong, lasting gastric acid suppression effect. TAK-438 is not mainly metabolized by protein CYP2C19, and it does not need to be activated by acid to inhibit protein pumps. As the drug enters the stomach at a high concentration, it will have the strongest suppressing effect at its first dosage, an effect which can last for up to 24 hours. TAK-438 is a stable acid, its formula is readily available and does not require optimized formulation (e.g. enteric coating), and its effective dosage amount does not vary dramatically between different patients. |
| Clinical Research | Compared to the traditional protein pump inhibitor Lansoprazole, Vonoprazan takes effect through competitive and reversible inhibition of K+ in protein pumps. Clinical and animal experiments show that Vonoprazan acts faster than PPI or H2 receptor blockers, has a stronger pH raising effect, can swiftly alleviate gastric symptoms, allows enzyme recovery after dissociation, and has few side effects. Multiple clinical trials have proved that in cases of erosive esophagitis, Vonoprazan prevents and treats gastric and duodenal ulcers. As a first-line response for eradicating helicobacter pylori, it has shown significant efficacy, higher than Lansoprazole and with minimal side effects. |
| Patents | Chemical compound patent: 200680040789.7, application date: August 29, 2006, expiration date: August 29, 2026, legal status: holding rights. Process patent: 201080018114.9, application date: February 24, 2013, expiration date: February 24, 2030. Legal status: In review – trial. Composition patent: WO2014003199, application date: June 26, 2013, no Chinese patent. |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately..

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved Design by Huishang Media