Product Name: | Estriol |
|---|---|
Synonyms | 1,3,5(10)-ESTRATRIEN-3,16A,17BETA-TRIOL 16ALPHA-HYDROXYESTRADIOL 3,16ALPHA,17BETA-TRIHYDROXY-1,3,5[10]-ESTRATRIENE ESTRIOL |
CAS | 50-27-1 |
MF | C18H24O3 |
MW | 288.38 |
EINECS | 200-022-2 |
Product Categories | Inhibitors; API; THEELOL; Hormone Drugs; Steroid and Hormone; Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals; Steroids; Hormone; Biochemistry; Hydroxysteroids |
Mol File |
Estriol Chemical Properties | |
|---|---|
Melting point | 280-282 °C(lit.) |
alpha | D25 +58° ±5° (0.04 g in 1 ml dioxane) |
Boiling point | 370.61°C (rough estimate) |
density | 1.27 |
refractive index | 58 ° (C=0.4, Dioxane) |
Fp | 9℃ |
storage temp. | -20°C Freezer |
solubility | Practically insoluble in water, sparingly soluble in ethanol (96 per cent). |
form | neat |
pka | pKa 10.38±0.02(H2O t=23±2 Iunspeci?ed) (Uncertain) |
Water Solubility | 3.2mg/L(25 ºC) |
Merck | 3707 |
BRN | 2508172 |
InChIKey | PROQIPRRNZUXQM-ZXXIGWHRSA-N |
CAS DataBase Reference | 50-27-1(CAS DataBase Reference) |
NIST Chemistry Reference | Estriol(50-27-1) |
EPA Substance Registry System | Estriol(50-27-1) |
Safety Information | |
|---|---|
Hazard Codes | T.F |
Risk Statements | 60-61-40-45-39/23/24/25-23/24/25-11 |
Safety Statements | 53-22-36/37/39-45-36/37-16 |
RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
WGK Germany | 3 |
RTECS | KG8225000 |
F | 8-10 |
HS Code | 29072990 |
Hazardous Substances Data | 50-27-1(Hazardous Substances Data) |
Toxicity | LD50 oral in rat: > 2gm/kg |
Safety Information | |
|---|---|
Hazard Codes | T.F |
Risk Statements | 60-61-40-45-39/23/24/25-23/24/25-11 |
Safety Statements | 53-22-36/37/39-45-36/37-16 |
RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
WGK Germany | 3 |
RTECS | KG8225000 |
| F | 8-10 |
HS Code | 29072990 |
Hazardous Substances | 50-27-1(Hazardous Substances Data) |
Data | |
Toxicity | LD50 oral in rat: > 2gm/kg |
Estriol Usage And Synthesis | |
|---|---|
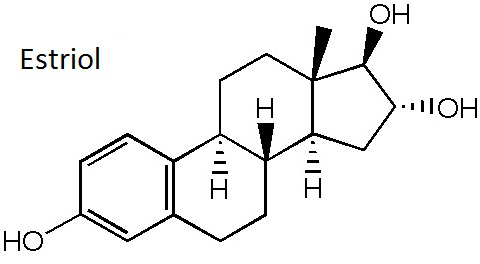
Natural hormone | Estriol belongs to natural hormone and is the metabolite of estradiol in vivo. It is mainly presented in the urine. Estrogen has a relative small activity with the oral activity being 6 times as high as estrone but being weaker than estradiol with non-carcinogenic effects. After its administration, the in vivo estradiol levels did not change. Estriol has selective action on the vaginal and cervical canal but has no effect on the uterus and endometrium entity. Animal experiments have shown that estriol has a stronger effect on vaginal epithelial keratosis than estradiol, thus being able to promote vaginal epithelial hyperplasia, superficial cells keratosis, mucosal angiogenesis and vaginal epithelial wound healing, but having a weak effect on the weight gain of mouse uterus. At the same time, estriol can enhance the function of cervix cell, causing the cervix muscle fiber hyperplasia and increasing the cervical elasticity and softness. In addition, estriol has feedback inhibition on the hypothalamus and pituitary but does not inhibit ovulation while only having significant impact on the corpus luteum and therefore can be used as the auxiliary drug in the medium-term labor induction and artificial abortion and for the treatment of various kinds of menopathy. Estriol also has significant effect on the hematopoietic system and can reduce vascular permeability and fragility. Therefore, it can be used for the treatment of various kinds of hemorrhage. It also has effect of rapidly increasing the peripheral leukocytes and generally begins to take effect at 1 to 3 days after treatment but with a shorter duration of action and is effective in treatment the leukopenia induced by radiotherapy and chemotherapy. Figure 1 is the formula of estriol |
Pharmacokinetics | It can be absorbed from the gastrointestinal tract and skin but is susceptible to damage upon oral administration and is therefore mainly subject to intramuscular injection and topical usage. |
Indications | It can be used for treating cervicitis, especially suitable for treating menopausal syndrome and senile vaginitis. |
Side effects | There are temporary breast swelling or lumps, menstrual disorders which can self-limiting and recovery after discontinuing the drug. |
Determination of estriol content | Estriol is produced through the hydroxylation of carbon 16 in dehydroepiandrosterone at fetal adrenal; it further enters into the placenta and the metabolism by the placental syncytiotrophoblast cells. |
Purpose and Function |
|
Precautions | 1. Pregnant and lactating women, patients of breast hyperplasia, breast lumps, gynecological cancer, and aplastic anemia patients should be disabled with minor patients being not suitable for using it. |
Drug Interactions | It can be used in combination with estradiol with competitive antagonism. |
Storage | It should be sealed upon shading for storage. |
Chemical Properties | It is white crystalline powder with the M.p. being 283 ℃, relative density being 1.27, and the specific rotation being [α] 25D + 34.4°(pyridine) and 25D + 58°± 5°(4%, dioxane). Its ethanol solution has maximum absorption at the wavelength of 280 nm. |
Uses | It belongs to estrogen drug. |
Production method | Take estrone as raw material; go through acetylation, epoxidation, and reduction to obtain the estriol products. Estrone [acetylation] → [16, 17-epoxidation] → [rearrangement] → [Restore] → [hydrolysis] → finished product of estriol. |
Chemical Properties | White or almost white, crystalline powder. |
Uses | 17b-estradiol metabolite, primary estrogen in urine |
Uses | A metabolite of Estradiol. An estrogenic metabolite considerably less potent than the hormone Estradiol |
Definition | ChEBI: A 3-hydroxy steroid that is estra-1,3,5(10)-trien-3-ol substituted by additional hydroxy groups at positions 16 and 17 (16alpha,17beta-stereoisomer). |
Brand name | Theelol (Parke-Davis). |
General Description | Estriol, estra-1,3,5(10)-triene-3,16 ,17 -triol, is available for compounding into several differentformulations for use in HRT. It can be used alone or in combinationswith estradiol (Bi-Est) or with estradiol and estrone(Tri-Est). |
Hazard | A carcinogen (OSHA). |
Safety Profile | Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. |
Purification Methods | Crystallise estriol from EtOH/ethyl acetate. Also purify it by countercurrent distribution with cyclohexane/EtOAc (1:1) and EtOH/H2O (1:1). The UV (EtOH) has max at 280nm ( 2,090 M-1cm-1). [Huffmann & Lott 71 719 1949, Leeds et al. J Am Chem Soc 76 2943 1954, Beilstein 6 IV 7550.] |
Estriol Preparation Products And Raw materials | |
|---|---|
Raw materials | 1,3,5(10)-Estratrien-3-ol-17-one |

If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately..

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved Design by Huishang Media