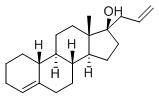
| Name |
|
|---|---|
| Synonyms | 13-methyl-17-prop-2-enyl-2,3,6,7,8,9,10,11,12,14,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ol 4-ESTREN-17-ALPHA-ALLYL-17-BETA-OL ALLYLESTRENOL 17-allyl-estr-4-en-17-beta-o 17-allylestr-4-en-17-beta-ol 17-alpha-allyl-17-beta-hydroxy-4-estrene 17-alpha-allyl-17-beta-hydroxy-delta(sup4)-estren 17-alpha-allyl-3-deoxy-19-nortestosterone 17-alpha-allyl-4-estren-17-beta-ol 17-alpha-allyl-4-oestrene-17-beta-ol 17-alpha-allylestr-4-en-17-beta-ol 17-alpha-allylhydroxy-19-nor-4-androstene 17-hydroxy-17-alpha-allyl-4-estrene 21-methylene-19-nor-17-alpha-preg-4-en-17-o 21-methylene-19-nor-17-alpha-preg-4-en-17-ol 3-deoxy-17-alpha-allyl-19-nortestosterone allyl-estreno allyloestrenol gestanin gestanol |
| CAS NO | 432-60-0 |
| EINECS | 207-082-9 |
| Molecular Weight | 300.48 |
| Molecular Formula | C21H32O |
| Product Categories | Steroid and Hormone; Inhibitors; Medicine intermediate |
| Mol File | 432-60-0.mol |
| Allylestrenol Chemical Properties | |
|---|---|
| density | 0.9914 (rough estimate) |
| Boiling point | 381.7°C (rough estimate) |
| Melting point | 79.5-80° |
| refractive index | 1.4800 (estimate) |
| pka | 14.95±0.40(Predicted) |
| CAS DataBase Reference | 432-60-0(CAS DataBase Reference) |
| Merck | 14,291 |
| Safety Information | |
|---|---|
| RTECS | KG7960000 |
| Allylestrenol Usage And Synthesis | |
|---|---|
| Originator | Alilestrenol ,Terapia |
| Uses | A synthetic steroid with progestational activity. |
| Manufacturing Process | To 145 ml of dry methylamine which is cooled to -20°C 1.5 g of lithium cut to small pieces are added. To the solution which is blue in color after 10-20 min, a solution of 3.0 g of oestradiol-3-methylether in 145 ml of absolute ether is added drop wise. Subsequently the reaction mixture is stirred at -10°C for 40 h, after which 50 ml of absolute ethanol are added. Then the methylamine is distilled off at law pressure. To the remaining solution 50 ml of ether and 50 ml of water are added. The water layer is separated and extracted with ether. The ethereal layer is washed with a 2 N hydrochloric acid solution, subsequently with a saturated sodium bicarbonate solution, and then with water. The ethereal solution is dried and evaporated to dryness. The resulting crude reaction product is dissolved in a mixture of benzene and petroleum ether (1:3) and chromatographed over aluminium oxide. The δ4-17β-hydroxy-oestrene obtained after chromatographic purification has a melting point of 80°-90°C and 95°-100°C after repeated crystallization from petroleum ether. A solution of 13.2 g of chromium trioxide in a mixture of 120 ml of water and 20 ml of acetic acid is added, with stirring, to a solution of 20 g of δ4-17β- hydroxy-oestrene in 400 ml of benzene. Subsequently the reaction mixture is vigorously stirred at room temperature for 16 h, after which the benzene layer is separated. The remaining aqueous layer is extracted a few times with benzene and the benzene extracts collected are then added to the separated benzene layer. The benzene extracts are successively washed with dilute sulfuric acid and water and then evaporated to dryness. The residue is crystallized from acetone, and the δ4-17β-oxo-oestrene, melting point 114°-116°C is obtained. To a mixture of 22.4 ml of absolute ether and 1.84 g of magnesium, a mixture of 2.72 ml of allyl bromide and 2.72 ml of absolute ether is added in nitrogen atmosphere. Subsequently a solution of 2 g of δ4-17β-oxo-oestrene in 30 ml of absolute ether is added to this reaction mixture, after which the whole is stirred for 4 h. Then the reaction mixture is poured into acidified ice water. The aqueous mixture is extracted with ether; the ether layer is separated, washed with water, dried over sodium sulfate and evaporated to dryness. The residue is recrystallized from a mixture of ether and petroleum ether, giving δ4-17β-hydroxy-17α-allyl-oestrene, melting point 79.5°-80°C. |
| Therapeutic Function | Progestin; Antiandrogen |
| Allylestrenol Preparation Products And Raw materials | |
|---|---|
| Raw materials | Lithium-->Magnesium-->Acetic acid-->Allyl bromide-->Chromium(VI) oxide-->Methylamine
|
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately..

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved Design by Huishang Media