| Name | DHEA |
|---|---|
| Synonyms | DHEA Astenile Deandros Diandron NSC 9896 Prestara Diandrone PRASTERONE 17-Chetovis Andrestenol D4000_SIGMA Psicosterone 17-Hormoforin ANDROSTENOLONE DHEA (1.03681) DHEA&DHEAAcetate Prasterone [INN] ANDRO-3B-OL-17-ONE Hydroxyandrostenone 3beta-hydroxy-5-and Dehydroepiandrostero Aster tataricus L.f. dehydroisoandosterone DEHYDROEPIANDOSTERONE Dehydroxyprogesterone DEHYDROEPIANDROSTERONE DEHYDROISOANDROSTERONE Prasteronum [INN-Latin] Prasterona [INN-Spanish] 5-Androsten-3B-ol-17-one 5-Dehydroepiandrosterone 5-Androsten-3β-ol-17-one Dehydrotransandrosterone 5-ANDROSTEIN-3B-OL-17-ONE TRANS-DEHYDROANDROSTERONE hydroxyandrost-5-en-17-one (+)-DEHYDROISOANDROSTERONE (3beta)-androst-5-en-17-on 5,6-Dehydroisoandrosterone 5,6-Dehydroisoandrostorone -Androsten-3beta-ol-17-one Dehydroepiandroserone(DHEA) Dehydroisoandrosterone DHEA (3-beta)-androst-5-en-17-on Epiandrosterone, 5-dehydro- 5-ANDROSTEN-3BETA-OL-17-ONE 3-HYDROXYANDROST-5-EN-17-ONE 5-ANDROSTENE-3BETA-OL-17-ONE Dehydroeplandrosterone(DHEA) 5,6-Didehydroisoandrosterone Dehydroepiandosterone / DHEA Dehydroepiandrosterone(DHEA) 3b-hydroxyandrost-5-en-17-one 3 B YDROXYANDROST-5-EN-17-ONE 3B-HYDROXY-5-ANDROSTEN-17-ONE (+)-Dehydroisoandrosterone,99% 3b-Hydroxyandrost-5-ene-17-one Dhea (+)-Dehydroisoandrosterone Dehydroepiandrosterone solution DEHYDROISOANDROSTERONE(DHEA)(AS) DEHYDROISOANDROSTERONE(DHEA)(RG) 3BETA-HYDROXY-5-ANDROSTEN-17-ONE 3BETA-HYDROXYANDROST-5-EN-17-ONE 3-beta-hydroxy-androst-5-en-17-on DEHYDROEPIANDROSTERONE,MICRONIZED 3beta-Hydroxyandrost-5-ene-17-one DEHYDROISOANDROSTERONE CRYSTALLINE (3β)-3-Hydroxyandrost-5-en-17-one Dehydroepiandrosterone (DHEA) (DMF) Androst-5-en-17-one, 3beta-hydroxy- Δ5-Androsten-3β-ol-17-one 3-β-3-Hydroxy-Androstene-5-en-17-One DehydroepiandrosteroneForBiochemistry (3-beta)-3-hydroxyandrost-5-en-17-one Dehydroepiandrosterone (DHEA) solution Dehydroepiandosterone(DHEA, Prasterone Dehydroepiandrosterone(AHEA,prasterone) Dehydroepiandrosterone Solution, 100ppm DHEA(DEHYDROEPIANDROSTERONE)&DERIVATIVES 3-hydroxy-,(3.beta.)-Androst-5-en-17-one Androst-5-en-17-one, 3-hydroxy-, (3beta)- Androst-5-en-17-one,3-hydroxy-,(3.beta.)- Androst-5-en-17-one, 3beta-hydroxy- (8CI) 5A-ANDROSTEN-3B-OL-17-ONE (PRASTERONE) (DHEA) Androst-5-en-17-one, 3-hydroxy-, (3-beta)- (9CI) (+)-Dehydroisoandrosterone Prasterone 3beta-Hydroxy-5-androsten-17-one (3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one 3β-Hydroxy-5-androsten-17-one, 5-Androsten-3β-ol-17-one, Dehydroepiandrosterone, Dehydroisoandrosterone, DHEA, Prasterone (3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-diMethyl-3,4,7,8,9,10,11,12,13,14,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one trans-Dehydroandrosterone,3β-Hydroxy-5-androsten-17-one, 5-Androsten-3β-ol-17-one, DHEA, Dehydroepiandrosterone, Dehydroisoandrosterone, Prasterone |
| CAS NO | 53-43-0 |
| MDL Number | MFCD00003613 |
| Molecular Weight | 288.42 |
| Molecular Formula | C19H28O2 |
| Dehydroepiandrosterone Chemical Properties | |
|---|---|
| Melting point | 149-151 °C(lit.) |
| alpha | 12 º (c=2, ethanol 96% 25 ºC) |
| density | 1.0484 (rough estimate) |
| Boiling poin | 370.65°C (rough estimate) |
| storage temp | Hormones |
| CAS DataBase Reference | 53-43-0 |
| refractive index | 1.4709 (estimate) |
| pka | 15.02±0.60(Predicted) |
| form | Fine Crystalline Powder |
| color | White |
| Water Solubility | 21.8mg/L(23.5 ºC) |
| Fp | 9℃ |
| Merck | 2871 |
| NIST Chemistry Reference | 5-Androstene-3«beta»-ol-17-one(53-43-0) |
| BRN | 2058110 |
| EPA Substance Registry System | Prasterone (53-43-0) |
| Safety Information | |
|---|---|
| Hazard Codes | Xi,T,F |
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36-24/25-45-36/37-16-7 |
| RTECS | BV8053000 |
| WGK Germany | 2 |
| HS Code | 29372900 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| Dehydroepiandrosterone Usage And Synthesis | |
|---|---|
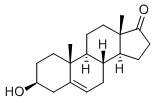
| description | Dehydroepiandrosterone (DHEA) is a steroid hormone that is a popular nonprescription oral “dietary supplement” used by men to enhance cognitive function, mood, libido, and athletic performance. Before 1994, DHEA was a prescription medicine. After the passage of the Dietary Supplement Health and Education Act of 1994, DHEA was reclassified as a nutritional supplement. Although sold over the counter in 25- and 50-mg strengths, DHEA is widely touted to maximize results at doses of 200 mg or higher. DHEA is banned by the International Olympic Committee and the National Collegiate Athletic Association as an anabolic agent. Limited information is available regarding potential harmful effects resulting from its supplemental use. |
| Indications and Usage | Dehydroepiandrosterone (DHEA,) chemical name 3β-hydroxy-5alpha-androstane-17-ketone, is an esterifying 3–β–hydroxy steroid retaining 5,6 cholesterol. A white crystalline powder, soluable in ethanol, ether, and benzene, and slightly soluable in chroloform and petroleum ether. Precipitates in digitalis. DHEA is an estrogen precursor secreted by the reticular layer of the human adrenal cortex. Prevents obesity, resists diabetes, fights cancer, fights cortical disease, and delays senility treats immune deficiencies, promotes the growth and differentiation of bone cells, and promotes the synthesis of protein. It also resists viral infections, improves memory, and relieves nervous tension. DHEA is the main ingredient in steroid hormone intermediates (such as norethindrone and bisacetylene, etc.) and in birth control, and is involved in the secretion of many adrenal hormones. It has undergone extensive clinical research on treating menopausal syndrom, chorionitis, coronary heart disease, gout, psoriasis, AIDS and so on. |
| Mechanisms of Action | DHEA has a thyroid stimulating effect inhibiting food and fat intake and reducing fat accumulation, etc. It improves glucose tolerance, increases insulin level and fights diabetes. It can enhance endocrine system actiity, reduce cortisol levels, and resist a variety of pathological processes. It can help the body obtain cortical antibodies. DHEA has a strong protective and synergistic effect when used to treat tumors, becuase it inhibits ribose 5-phosphate. Inhibits cancer by inhibiting excessive mitochondria (NADPH) and ribose 5-phosphate esters. Regulates the growth of pancreatic cancer cells by regulating the concentration of estrogen in blood plasma. A decline in GnRH gene expression leads to aging, and DHEA can restore GnRH neuronal activity, stopping or improving certain diseases associated with declines in DHEA by stimulating GnRH biosynthesis. Restores impaired immune response, improves T- and B-cell function, and plays an important role in enhancing the physiological activity of insulin-like growth factor (IGF-1,) and is a potentially useful drug for immunodeficiency. DHEA alone cannot directly affect the growth and differential of osteoblasts, but it can do so by influencing 1,25-dihydroxyvitamin D3. The effects of DHEA on bone mass depend on the presence and form of sex hormones in bone cells and their endrocine effects on osteoblasts. DHEA is an anabolic protein hormone which promotes protein synthesis. According to the findings of Marrero and others, feeding DHEA (0.45%) to mice increased liver weight, increasing liver mitochondria by guiding liver protein restoring RNA and protein synthesis. |
| Chemical Properties | white fine crystalline powder |
| Originator | Aslera ,Genelabs Technologies, Inc. |
| Occurrence | DHEA is naturally occurring in yam (see Wild Yam, p. 596-597). |
| Uses | Major secretory steroidal product of the adrenal gland; secretion progresively declines with aging. May have estrogen-or androgen-like effects depending on the hormonal milieu. Intracellularly converted to androstenedione. It is used in treatment of menopausal syndrome. |
| Uses | adrenocortical hormone, antidepressant |
| Manufacturing Process | To a solution of 1 gram of 16-dehydropregnenolon-3β-acetate in 10 ml pyridine is added 0.22 gram of hydroxylamine hydrochloride, and the mixture is allowed to stand at room temperature for four days. One gram of 16dehydropregnenolon-3β-acetate oxime is dissolved in 30 ml of hot dioxane, and then the solution is cooled in an ice bath until about one-half of the dioxane has solidified. Then 1 gram of phosphorus pentachloride is added and the mixture is shaken until all the dioxane has melted. The mixture is maintained at 35°C, for seventy-five minutes, then an excess of ice is added and the solution is again allowed to stand at 35°C. After about thirty minutes, a solution of 5 ml of concentrated hydrochloric acid in 10 ml of water is added, and the mixture is diluted with water, extracted with ether and the ethereal extract washed with dilute sodium hydroxide solution. The ether is removed on a steam bath and the residue is worked up to yield dehydroisoandrosterone. |
| Brand name | 17-chetovis 17-hormoforin;Cetavister;Climatost;Dastonil;Dha-s (prasterone);Gynodian;Longevital 5000;Maxepa;Mentalormon;Mylis;Neurocotex;Psicosterone;Ro 66827;Sh 833;Ultrapla. |
| Therapeutic Function | Glucocorticoid |
| World Health Organization(WHO) | The World Health Organization has no information further to the above regarding preparations containing prasterone or to indicate that such preparations remain available. |
| Safety Profile | An experimental teratogen. Experimental reproductive effects. Questionablecarcinogen with experimental neoplastigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.

If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately..

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved Design by Huishang Media