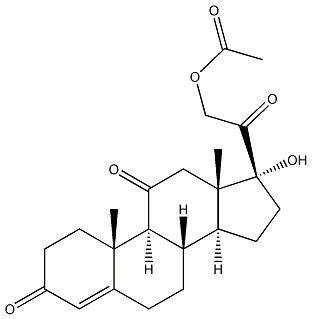
| Name | Cortisone acetate |
|---|---|
| Synonyms | 11,20-trione,17,21-dihydroxy-pregn-4-ene-21-acetate; 11-dehydro-17-hydroxycorticosterone-21-acetate; 11-dehydro-17-hydroxycorticosteroneacetate; 17,21-dihydroxypregn-4-ene-3,11,20-trioneacetate; 4-PREGNEN-17ALPHA,21-DIOL-3,11,20-TRIONE ACETATE; 4-PREGNEN-17,21-DIOL-3,11,20-TRIONE 21-ACETATE; 4-PREGNENE-17ALPHA,21-DIOL-3,11,20-TRIONE 21-ACETATE; 17ALPHA,21-DIHYDROXY-4-PREGNENE-3,11,20-TRIONE 21-ACETATE 17,21-DIHYDROXYPREGN-4-ENE-3,11,20-TRIONE 21 ACETATE 17ALPHA,21-DIHYDROXY-4-PREGNENE-3,11,20-TRIONE 21-ACETATE 21-acetoxypregnen-17a-ol-3,11,20-trione 4-PREGNEN-17,21-DIOL-3,11,20-TRIONE 21-ACETATE 4-PREGNEN-17ALPHA,21-DIOL-3,11,20-TRIONE ACETATE 4-PREGNENE-17ALPHA,21-DIOL-3,11,20-TRIONE 21-ACETATE ACETIC ACID 2-((8S,9S,10R,13S,14S,17R)-17-HYDROXY-10,13-DIMETHYL-3,11-DIOXO-2,3,6,7,8,9,10,11,12,13,14,15,16,17-TETRADECAHYDRO-1H-CYCLOPENTA[A]PHENANTHREN-17-YL)-2-OXO-ETHYL ESTER CORTISONE 21-ACETATE CORTISONE ACETATE DELTA4-PREGNEN-17ALPHA-21-DIOL-3, 11, 20-TRIONE-21-ACETATE KENDALL'S COMPOUND 'E' ACETATE 11,20-trione,17,21-dihydroxy-pregn-4-ene-21-acetate 11-dehydro-17-hydroxycorticosterone-21-acetate 11-dehydro-17-hydroxycorticosteroneacetate 17,21-dihydroxypregn-4-ene-3,11,20-trioneacetate 20-trione,21-(acetyloxy)-17-hydroxy-pregn-4-ene-11 21-(acetyloxy)-17-hydroxypregn-4-ene-3,11,20-trione 21-acetoxy-17,alpha-hydroxy-3,11,20-triketopregnene-4 21-acetoxy-17,alpha-hydroxypregn-4-ene-3,11,20-trione acetatecortisone |
| CAS NO | 50-04-4 |
| EINECS | 200-006-5 |
| Molecular Weight | 402.48 |
| Molecular Formula | C23H30O6 |
| Product Categories | Biochemistry; Hydroxyketosteroids; Steroids; Inhibitors; CORTONE; Hormone Drugs |
| Mol File | 50-04-4.mol |
Cortisone acetate Chemical Properties | |
|---|---|
| Melting point | 237-240 °C(lit.) |
| alpha | D25 +164° (c = 0.5 in acetone); D25 +208 to +217° (dioxane) |
| refractive index | 212 ° (C=1, MeOH) |
| storage temp | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in dioxan, sparingly soluble in acetone, slightly soluble in ethanol (96 per cent) and in methanol. |
| form | neat |
| Water Solubility | 19mg/L(25 ºC) |
| Merck | 14,2539 |
| BRN | 2067543 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-04-4(CAS DataBase Reference) |
| EPA Substance Registry System | Cortisone acetate (50-04-4) |
| Safety Information | |
|---|---|
| Safety Statements | 22-36/37-24/25 |
| WGK Germany | 3 |
| RTECS | GM9140000 |
| HS Code | 32041200 |
| Cortisone acetate Usage And Synthesis | |
|---|---|
| Chemical Properties | solid |
| Originator | Cortone Acetate,MSD,US,1950 |
| Uses | Cortisone Acetate is a glucocorticoid. Cortisone Acetate is an antiinflammatory agent. Cortisone Acetate is bioavailable and readily converted to the therapeutically active form, Hydrocotisone (H71461 5). |
| Manufacturing Process | The following technique is described in US Patent 2,541,104. A solution of 2.0 g of 3(α)-hydroxy-21-acetoxy-11,20-diketo-pregnane, which can be prepared as described in Helv. Chim. Acta 27, 1287 (1944), is treated in a mixture of 25 cc of alcohol and 6.4 cc of acetic acid at 0°C with 6.0 g of potassium cyanide. The solution is allowed to warm to room temperature and after 3 hours is diluted with water. The addition of a large volume of water to the alcohol-hydrogen cyanide mixture precipitates a gum which is extracted with chloroform or ethyl acetate. The extract is washed with water, and evaporated to small volume under reduced pressure. The crystalline precipitate (1.3 g) consists of 3(α),20-dihydroxy-20-cyano-21-acetoxy-11-keto-pregnane; dec. 175° to 185°C. A solution of 0.60 g of chromic acid in 1.2 cc of water and 11 cc of acetic acid is added to a solution containing about 1.2 g of 3(α),20-dihydroxy-20-cyano- 21-acetoxy-11-ketopregnane at room temperature. After 1 hour, water is added and the product, which precipitates, is filtered and recrystallized from ethyl acetate to produce 3,11-diketo-20-hydroxy-20-cyano-21-acetoxypregnane; dec. 214° to 217°C. 0.40 cc of phosphorus oxychloride is added to a solution containing about 950 mg of 3,11-diketo-20-hydroxy-20-cyano-21-acetoxy-pregnane dissolved in 3 cc of pyridine. After standing at room temperature for 24 hours, the solution is poured into water and dilute hydrochloric acid, extracted with benzene and concentrated to dryness. The crude product, after chromatography gives one main constituent, namely δ17-3,11-diketo-20-cyano-21-acetoxy-pregnene; MP 189° to 190°C. A solution of 1.0 g of δ17-3,11-diketo-20-cyano-21-acetoxy-pregnene in 10 cc of benzene is treated with 1.0 g of osmium tetroxide and 0.43 g of pyridine. After standing at room temperature for 18 hours, the resulting solution is treated successively with 50 cc of alcohol, and with 50 cc of water containing 2.5 g of sodium sulfite. The mixture is stirred for 30 hours, filtered, and the filtrate acidified with 0.5 cc of acetic acid and concentrated to small volume in vacuo. The aqueous suspension is then extracted four times with chloroform, the chloroform extracts are combined, washed with water and concentrated to dryness in vacuo. Recrystallization of the residue from acetone gives 9°C. This compound is then treated with acetic anhydride and pyridine for 15 minutes at room temperature to produce 3,11,20-triketo-17(α)-hydroxy-21-acetoxypregnane or cortisone acetate. |
| Therapeutic Function | Glucocorticoid |
| General Description | Cortisone acetate, 21-(acetyloxy)-17-hydroxypregn-4-ene-3,11,20-trione, is the 21-acetate of naturally occurring cortisone with good systemicanti-inflammatory activity and low-to-moderate salt-retentionactivity after its in vivo conversion to hydrocortisoneacetate. This conversion is mediated by 11β-hydroxysteroiddehydrogenase. It is used for the entire spectrum of uses discussedpreviously under the heading, “Therapeutic Uses ofAdrenal Cortex Hormones”—collagen diseases, Addisondisease, severe shock, allergic conditions, chronic lymphocyticleukemia, and many other indications. Cortisone acetateis relatively ineffective topically, mainly because itmust be reduced in vivo to hydrocortisone. Its plasma halflifeis only about 30 minutes, compared with 90 minutes to3 hours for hydrocortisone. |
| Purification Methods | Crystallise -1cortisone-21-acetate from acetone or CHCl3. The UV has 15,800 M-1cm at 238nm in dioxane. [Sarett J Biol Chem 162 601 1946, Beilstein 8 III 4058, 5 IV 3481.] |
| Hydrocortisone acetate Preparation Products And Raw materials | |
|---|---|
| Raw materials | Hydrogen peroxide-->Nickel-->Chromium(VI) oxide-->16-Dehydropregnenolone acetate-->Potassium Acetate-->16a,17a-Epoxyprogesterone-->Chromic acid-->Saponin-->CORTISONE-->16-Dehydropregnenolone-->POTASSIUM CYANIDE-->Acetic acid-->Osmium tetroxide
|
| Preparation Products | Hydrocortisone-->Prednisone-->Prednisone 21-acetate |
| Package method |
|---|

FAQ
MOQ: 100 gram
Pack material: Plastic bag + Shockproof film + shockproof envelope + Cartons.
Shipment: By express to buyers’ door. 100% make sure delivery.
Payment: TT/ Western Union/BTC/ETV/VISA and so on, please contact by email.
Shipment time: Within three working days after payment. Usually need ten days to arrive buyers’ address. Resend if lost.

If you have any questions or ask for a quote, please submit your information here and we will respond to you immediately..

Boldenone, Oxymetholone, Drostanolone, Testosterone, Nandrolone, Trenbolone
Copyright © 2008-2022 J·S Biology Co.,LTD All Rights Reserved Design by Huishang Media